Our laboratory's research focus is aimed on understanding how stem/progenitor cell maintenance and commitment/differentiation are achieved through regulatory mechanisms involved in cell cycle regulation, transcriptional control, and chromatin organization. We further aim to investigate how deregulation of these processes leads to cancer and neurodevelopmental diseases like microcephalyand hydrocephalusin humans.
Towards this direction we are studying the role of the Geminin superfamily, a family that contains three genes (Geminin, GemC1 and McIdas) involved in the regulation of proliferation and cellular differentiation through interactions with regulators of DNA replication origin licensing, transcription factors and chromatin modifying complexes. Based on our previous experience together with tools and reagents already generated, we are using conditional knock out mice, stem cell cultures and high-throuput approaches to study the role of Geminin in the regulation of self-renewal and fate commitment/differentiation decisions stem and progenitor cells.
CURRENT PROJECTS
Adult Neurogenesis And Ependymal cells Biology
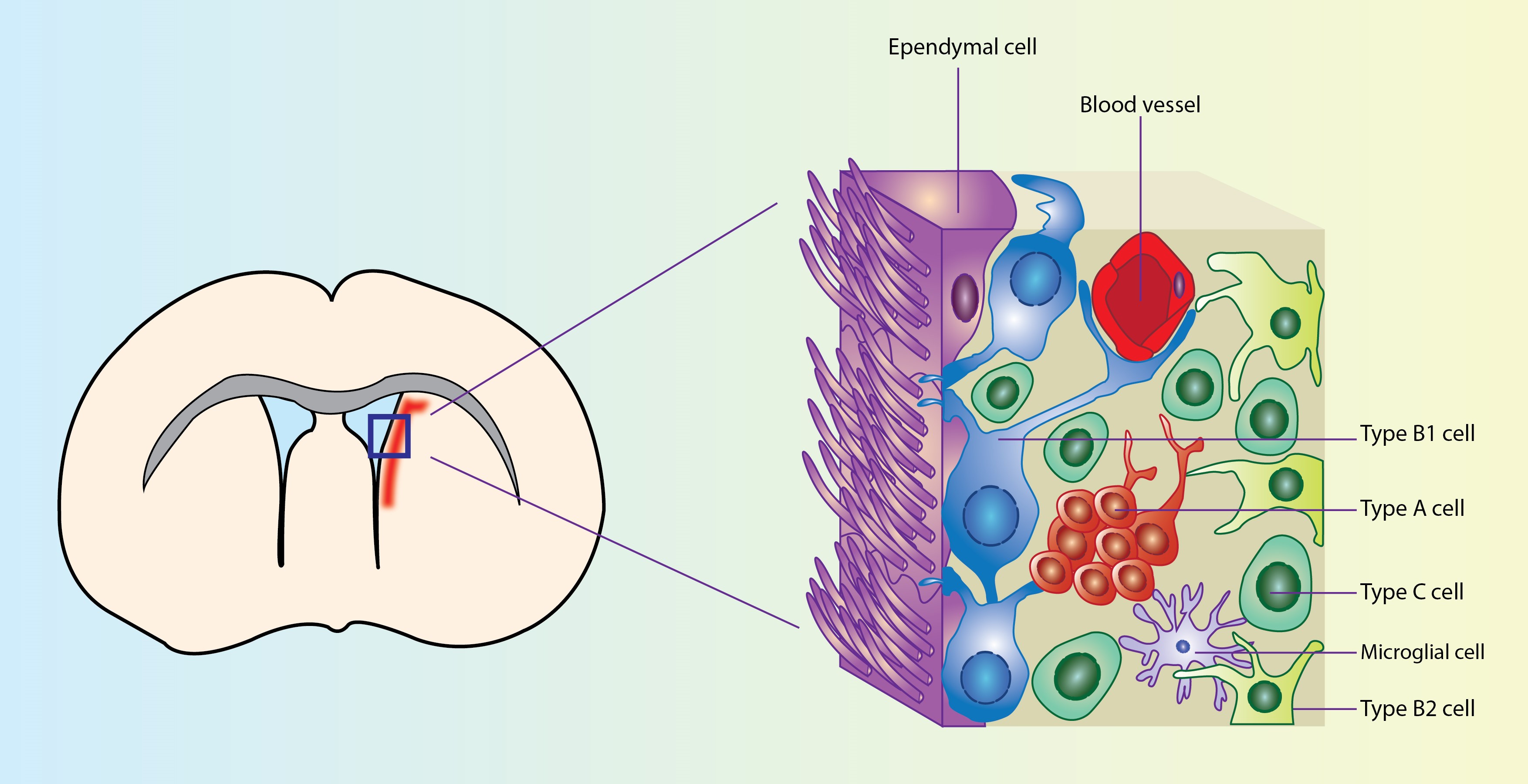 The subventricular zone (SVZ) is the largest germinal niche in the adult mammalian brain. In the SVZ niche two main cellular populations can be identified: the adult neural stem cells (NSCs) with self-renewal capacity and multi-lineage potency and the nondividing multiciliated ependymal cells. Ependymal cells carry multiple motile cilia which contribute to cerebrospinal fluid circulation, while they provide structural and functional support to the adult NSCs. Loss and dysfunction of the ependymal cells has been linked to the formation of hydrocephalus, a clinical entity defined as abnormal circulation/accumulation of cerebrospinal fluid in the brain ventricles, both in mice and humans.
The subventricular zone (SVZ) is the largest germinal niche in the adult mammalian brain. In the SVZ niche two main cellular populations can be identified: the adult neural stem cells (NSCs) with self-renewal capacity and multi-lineage potency and the nondividing multiciliated ependymal cells. Ependymal cells carry multiple motile cilia which contribute to cerebrospinal fluid circulation, while they provide structural and functional support to the adult NSCs. Loss and dysfunction of the ependymal cells has been linked to the formation of hydrocephalus, a clinical entity defined as abnormal circulation/accumulation of cerebrospinal fluid in the brain ventricles, both in mice and humans.
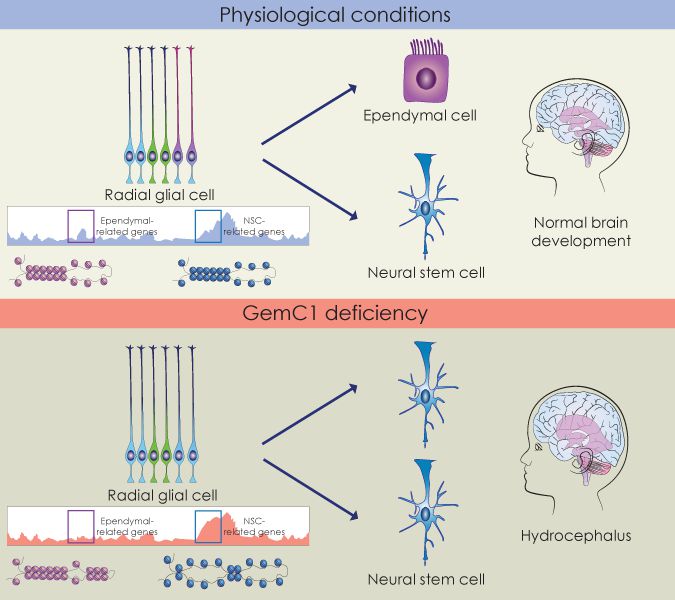
Our research focuses on understanding how neural stem cells' fate choices are coordinated at the molecular level. We wish to identify the molecular pathways that govern differentiation of neural progenitor cells in the brain into distinct cellular types of the adult brain. Ultimately, by deciphering the mechanisms that are implicated in this process we will be able to understand how deregulation of these mechanisms leads to neurological disorders like hydrocephalus.
A novel mutation in GemC1 as a cause of hydrocephalus in humans. The absence of GemC1 leads to a neural stem cell-like fate at the expense of ependymal cells. GemC1 remodels the chromatin landscape of neural progenitor cells
Genomic Instability In Cancer And Neurodevelopmental Diseases
Strict regulation of DNA replication is an important procedure since cases of its dysfunction lead to development of replication stress, a frequent source of genomic instability. Replication stress-induced genomic instability has been widely associated with tumorigenesis and is lately considered to affect the innate immune system activation.
The replication licensing factor Cdt1 and its inhibitor Geminin are key regulators of DNA replication. Geminin binds to Cdt1 preventing relicensing of replication during the same cell cycle. According to previous studies of our research group, changes in expression levels of these proteins cause re-replication and are linked to signs of DNA damage contributing to the development of murine lung and colon carcinogenesis. We aim to elucidate the mechanism activated in inflammatory responses during genomic instability induced by the deregulation of Geminin and Cdt1 expression levels. One of our long-term goals is to provide insight on how inflammation determines the cancer progression, as well as to reveal molecular targets for anticancer therapy.
Geminin ablation in vivo enhances tumorigenesis through increased genomic instability
The diverse populations of neural stem cells (NSCs) that emerge during cortical development exhibit distinct cell cycle kinetics and differential regulation of the DNA replication licensing and initiation processes. Neuroepithelial cells are characterized by a shorter cell cycle compared to Radial Glial cells that permits their fast proliferation. During the G1 phase origins of replication are licensed by the formation of a competent pre-replicative complex (Pre-RC). Higher expression of licensing factors (ORC, Cdc6, Cdt1 and MCMs) is required for the efficient licensing of origins in NECs that exhibit a shorter G1 phase.
Origins of replication are licensed during the G1 phase by the formation of a competent pre-replicative complex (Pre-RC). Impaired licensing and initiation of DNA replication lead to defective brain development and microcephaly. Reduced expression of licensing factors results in decrease of licensed origins that further causes incomplete initiation of DNA replication. Aberrant licensing directly affects the successful duplication of the genome due to under-replication or over replication. Under these conditions, the proliferation of NSCs is compromised resulting in severe brain malformations.




