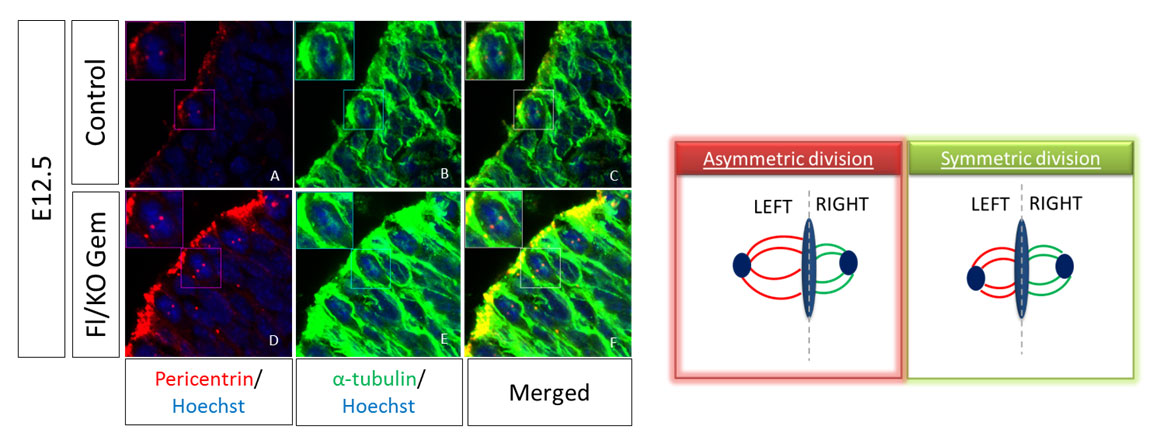
The size of the mitotic spindle during mitosis can give us information about the type of the division. During active neurogenesis Apical Radial Glia cells perform two types of divisions in order to self-renew or to differentiate, termed symmetric or asymmetric divisions respectively. For the evaluation of the division type we have to measure the volume of each spindle pole and calculate the difference between the two poles. This difference is the SSA index; high SSA index shows asymmetric divisions while a small number shows a symmetric division. To visualize the mitotic spindle in vivo, we perform immunofluorescence (IF) staining on cryosections of mouse embryos at E12.5 dpc with antibodies against α-tubulin and pericentrin, two main proteins of the spindle. To analyze, we take pictures in thin stacks 0,2μm and calculate the volume of each pole and subsequently the SSA index.
 |
Experimental Procedure
Collection of tissues
- Collect the embryos during mid-neurogenesis between embryonic day 12.5 (E12.5) and embryonic day 16.5 (E16.5) for analysis.
- Pregnant females are anesthetized with the use of CO2 and are sacrificed with cervical dislocation. Reveal the embryos with a caesarian section and transfer the uterus in clean PBS. Perform all of the dissection steps at RT in order to prevent microtubule depolymerization.
- Remove the uterine horns and the yolk sacs from each embryo and chop the head with a single cut using forceps. Was 3 times for 5min (3x5min) with PBS.
- Fix embryo heads in 2% PFA for 2h RT rocking. Wash 3x5min with PBS.
- Transfer embryos in 10% sucrose until they dive in completely and then remove the solution and add 20% sucrose. Again wait until they dive in completely.
- Freeze the tissues in OCT and prepare cryosections 20-25μm thick.
Staining
- Incubate the sections with PBS for 15min to hydrate and then incubate with PBS-Triton 0.5% for another 15min.
- Block the sections by incubating in blocking solution (1% Normal Goat Serum, 2% Bovine Serum Albumin in PBS-Triton 2%) for 1h.
- Apply the primary antibodies overnight at 4oC and make sure that all the sections are covered by antibodies solution and that the chamber is well humidified. Antibodies are diluted in blocking solution. Wash the sections 3x15min with PBS
- Incubate with the secondary antibodies for 2h RT and then wash 3x15min with PBS. From that step protect sections from light.
- To counterstain the nuclei incubate with Hoechst solution for 5min RT and the wash 3x15min with PBS.
- Remove the excess of PBS with a tissue and put glass coverslips using Mowiol as mounting medium.
- Keep sections at 4οC protected from light.
Confocal Imaging
- Dividing aRGCs are always located in the ventricular zone lining the lateral ventricles of the brain. Detect a dividing cell during metaphase (you will recognize the metaphase from the characteristic morphology of the nucleus during that stage of mitosis) and acquire photos of 0.2-0.5 μm z intervals.
- Analyze the photos in ImageJ and calculate the volume for each pole of the spindle.
Protocol was kindly provided by Dr. Colette Dehay lab.
Mitotic spindle asymmetry: a Wnt/PCP-regulated mechanism generating asymmetrical division in cortical precursors., Delaunay et al., Cell Reports 2014.
This work was supported by Aristeia II, GEMCCTR “Self-renewal and differentiation decisions in neural stem cells: Geminin, cell cycle control and transcriptional regulation”




