Dissection and Fixation
1. The animal is sacrificed by cervical dislocation and the head is cut off. The brain is extracted and placed into a dissecting dish filled with 0.1 M PBS.
2. The brain is divided and a coronally-oriented cut is then made at the posterior-most aspect of the interhemispheric fissure, allowing the caudal hippocampus to be visualized in cross-section.
3. The hippocampus is released from the overlying cortex using small strokes
4. Subsequently, the cortex is dissected away with small cuts by visualizing the interface between the corpus callosum and the SEZ.
Note: Simply cut along this interface staying on the callosal side to avoid damaging the SVZ.
5. Finally, the lateral wall is completely exposed by removing any overhanging cortex dorsally and the thalamus ventrally.
The wholemount is transferred (ventricle side up) from the dissection dish into a 24-well plate filled with 4% PFA with 0.1% Triton-X100 for an overnight fixation at 4°C.
Immunostaining
7. The following morning, PFA is aspirated from the 24-well plate and the wholemounts are washed 3 times for 5 minutes each in 0.1 M PBS with 0.1% Triton-X100.
Note: The ventricle side of the wholemount must be facing up at all times.
8. Wholemounts are incubated for 1 hour at room temperature in blocking solution, containing 10% normal goat serum in 0.1 M PBS with Triton-X100.
Note: The concentration of TritonX100 depends on the depth that the antigens are located. For example 2% Triton-X100 is suggested for staining antigens that require deeper antibody penetration into the tissue, whereas 0.5% Triton-X100 is preferred for antigens found in the apical surface of ependymal cells.
9. The blocking solution is removed and the primary antibodies (diluted in the same blocking solution) are added. The whole mounts are incubated for 48 hours at 4°C.
10. The primary antibodies are washed off initially by 2 quick rinses in PBS with 0.1% Triton-X100. Afterwards, 3 additional washes for 20 minutes each at room temperature are made.
11. The secondary antibodies are diluted in the blocking solution and added to the wholemounts for 48hours at 4°C (in the dark).
12. Secondary antibodies are washed off the wholemount using the same washes performed for primary antibodies.
For nuclear staining, hoechst is diluted in PBS for 30 minutes at room temperature and a final wash with PBS is made.
Mounting Immunostained Wholemounts
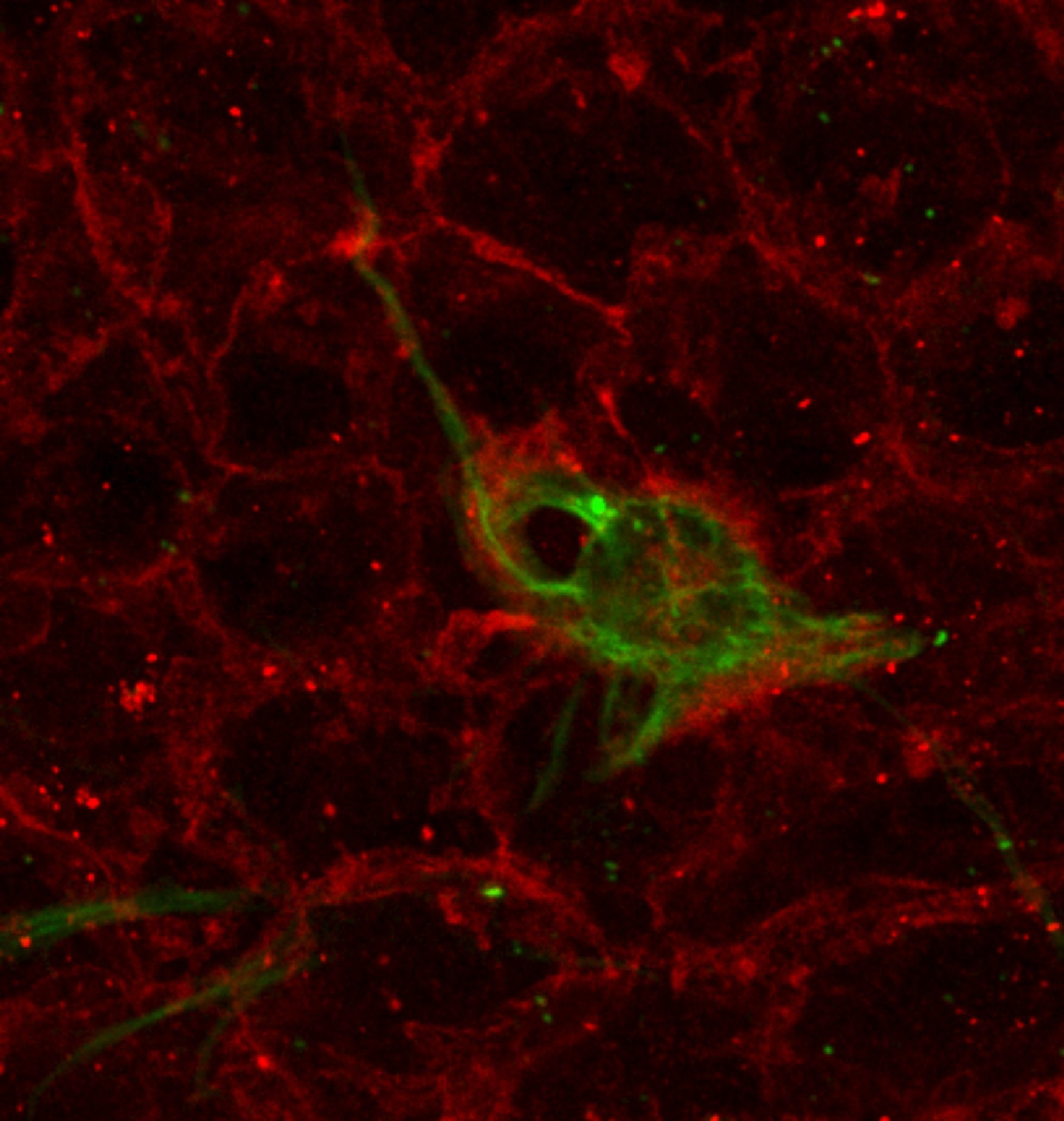
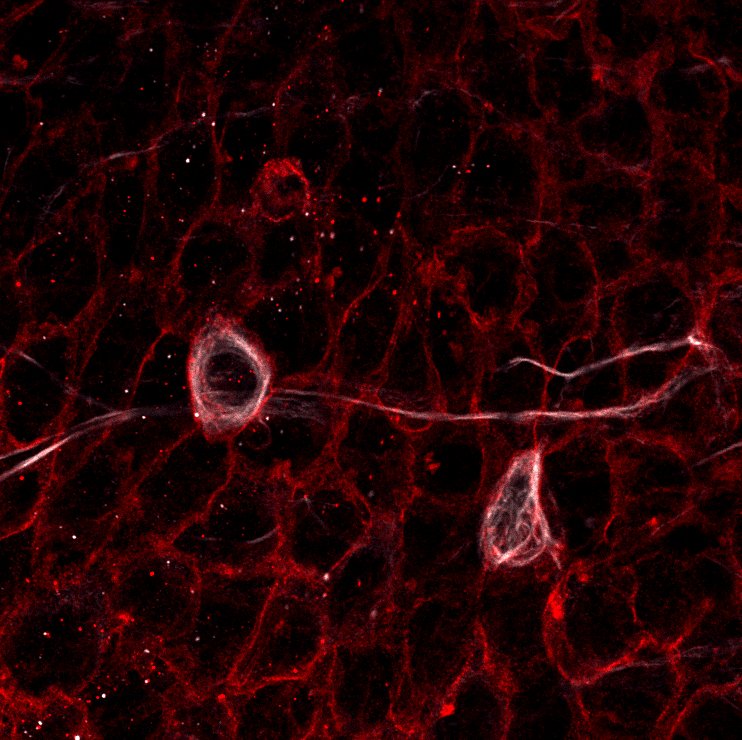
13. The wholemount is sub-dissected to preserve only the lateral wall of the lateral ventricle as a sliver of tissue 200-300 um thick. In detail, the dorsal cortex is completely removed and the dorsal surface of the wholemount is facing up, allowing to separate the underlying striatum and SEZ. Afterwards, the SEZ is sliced off from anterior to posterior across the lateral wall (Note that the orientation of the cuts made must remain parallel to the ventricular surface at all times).
14. After completely separating the lateral wall from the underlying striatum, all other surrounding tissues must be removed.
15. Finally, the SEZ is put in the center of a microscope slide and mounted using MOWIOL and a coverslip. The slides are then stored flat in a slide book at 4°C for 1-2 days before imaging to allow the coverslips to settle.
Protocol from Mirzadeh et al., Cell Stem Cell (2008)
This work was supported by Aristeia II, GEMCCTR “Self-renewal and differentiation decisions in neural stem cells: Geminin, cell cycle control and transcriptional regulation”
 |
 |




