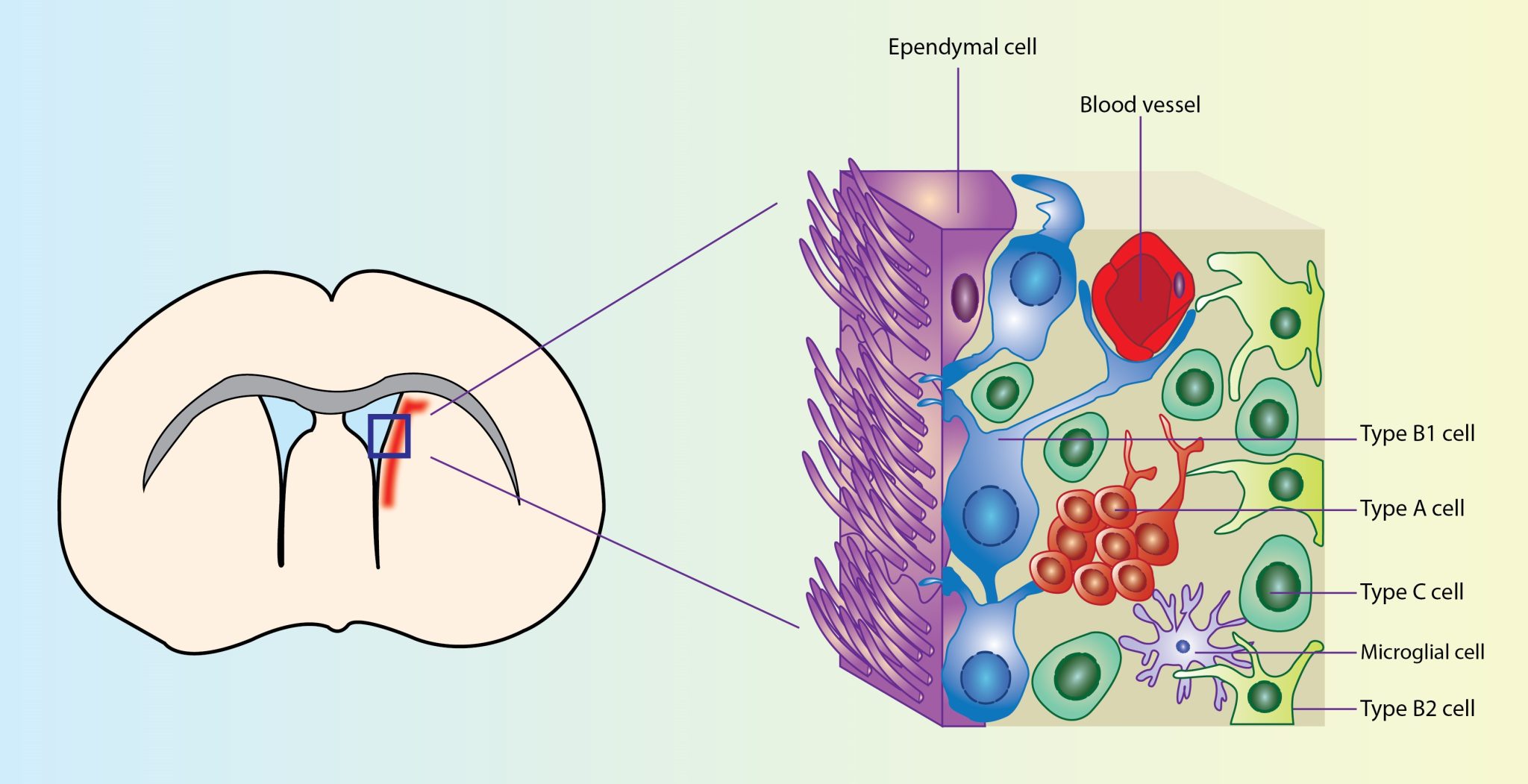
The subventricular zone (SVZ) is the largest germinal niche in the adult mammalian brain. In the SVZ niche two main cellular populations can be identified: the adult neural stem cells (NSCs) with self-renewal capacity and multi-lineage potency and the nondividing multiciliated ependymal cells. Ependymal cells carry multiple motile cilia which contribute to cerebrospinal fluid circulation, while they provide structural and functional support to the adult NSCs. Loss and dysfunction of the ependymal cells has been linked to the formation of hydrocephalus, a clinical entity defined as abnormal circulation/accumulation of cerebrospinal fluid in the brain ventricles, both in mice and humans.
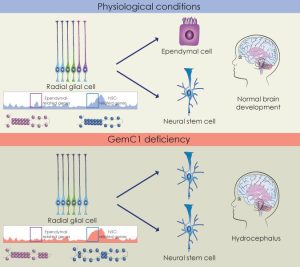
Our research focuses on understanding how neural stem cells’ fate choices are coordinated at the molecular level. We wish to identify the molecular pathways that govern differentiation of neural progenitor cells in the brain into distinct cellular types of the adult brain. Ultimately, by deciphering the mechanisms that are implicated in this process we will be able to understand how deregulation of these mechanisms leads to neurological disorders like hydrocephalus.
A novel mutation in GemC1 as a cause of hydrocephalus in humans. The absence of GemC1 leads to a neural stem cell-like fate at the expense of ependymal cells. GemC1 remodels the chromatin landscape of neural progenitor cells.